Enterprise Workflow Management
STATLIA MATRIX streamlines and manages your assay workflow, data management, and regulatory compliance as only an enterprise software system can, allowing you to focus on running your assays.See your data in STATLIA MATRIX.
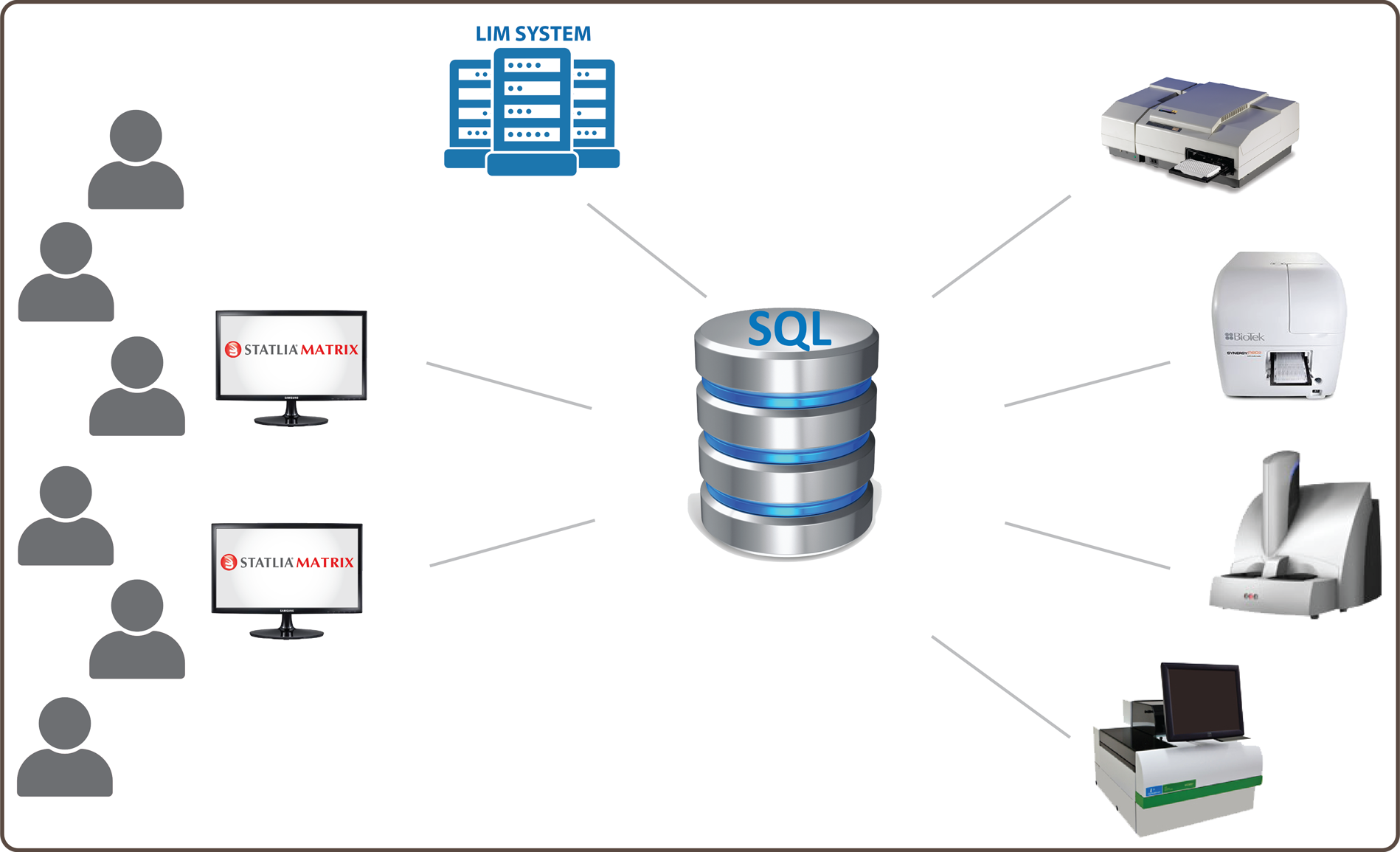
SQL Database Structure Is Key to Managing Assay Data and Workflow
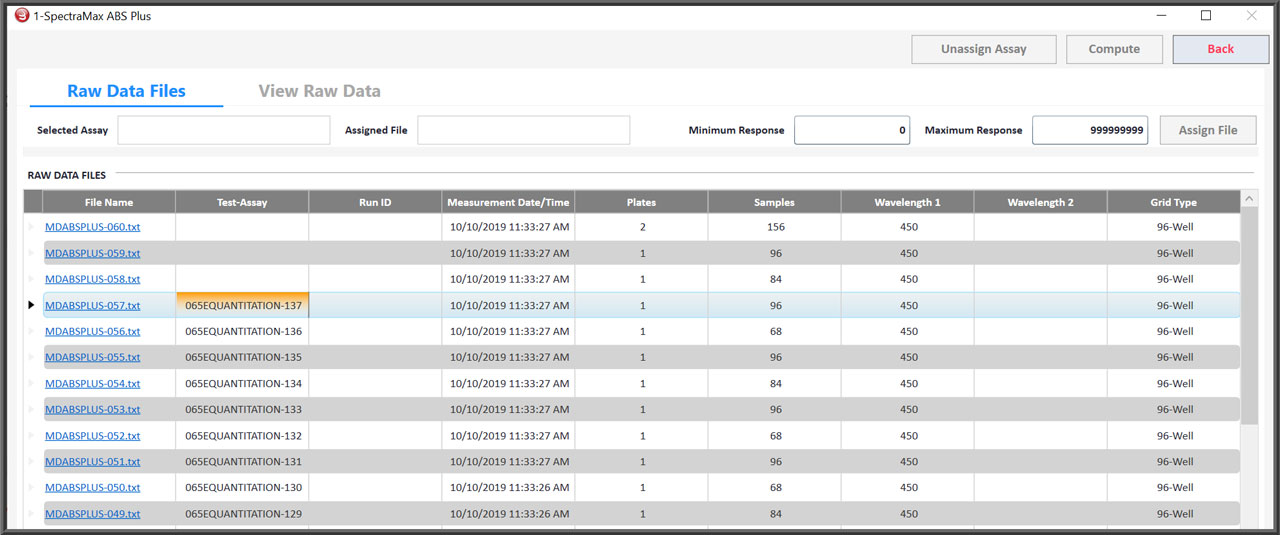
- STATLIA MATRIX is structured around a robust Microsoft SQL Server database that secures, organizes, and provides easy access to all of your assays, raw data, assay data, reports, approvals, and more.
- Only one software system is needed to standardize all immunoassay, potency bioassay, and immunogenicity testing technologies.
- Only one software system is needed for all of your detector instruments.
- Only one software system to validate for all technologies and all detector instruments – with the fully automated STATLIA MATRIX IQ/OQ Validation Package.
- Only one software system needed to enable your GxP bioanalytical laboratory to have complete regulatory compliance with 21 CFR Part 11.

STATLIA MATRIX Centralizes, Standardizes, and Streamlines Laboratory Workflow
GxP Enterprise System Structured Around Secure Microsoft SQL Server Database
- Standardize all of your immunoassay, potency bioassay, and immunogenicity testing technologies to a single system that utilizes the most advanced analysis and workflow management in the industry.
- Install on a client-server local area network (LAN), a cloud with virtual desktop interfaces (VDIs), or remote access VDIs (such as CITRIX).
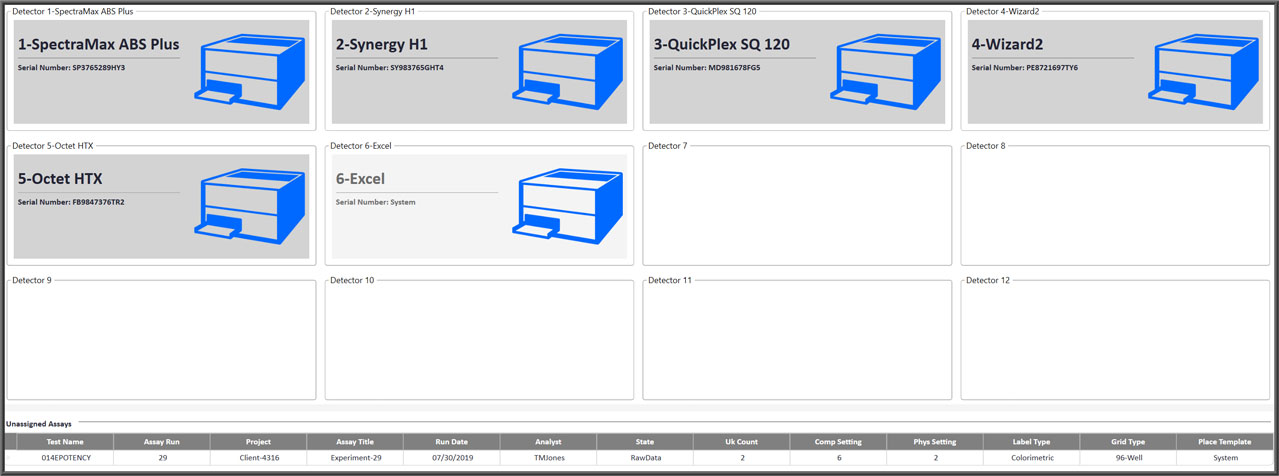
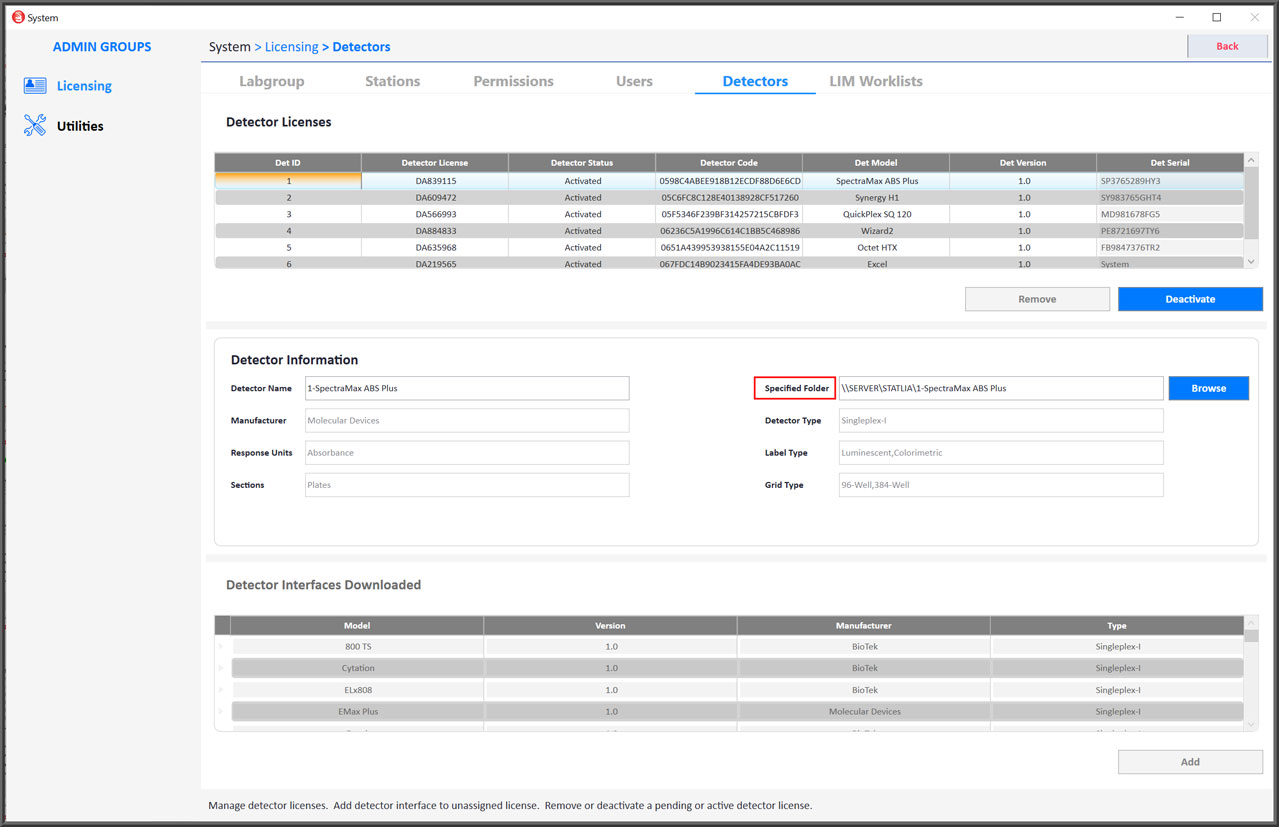
- All detector instrument interfaces from all testing technologies are easily installed and ready to collect data.
- Add as many computer stations or VDIs as your laboratory needs, and easily add additional stations or VDIs as your laboratory needs grow.
- Users can be assigned to individual security groups with specified permissions, and users can be added or changed.
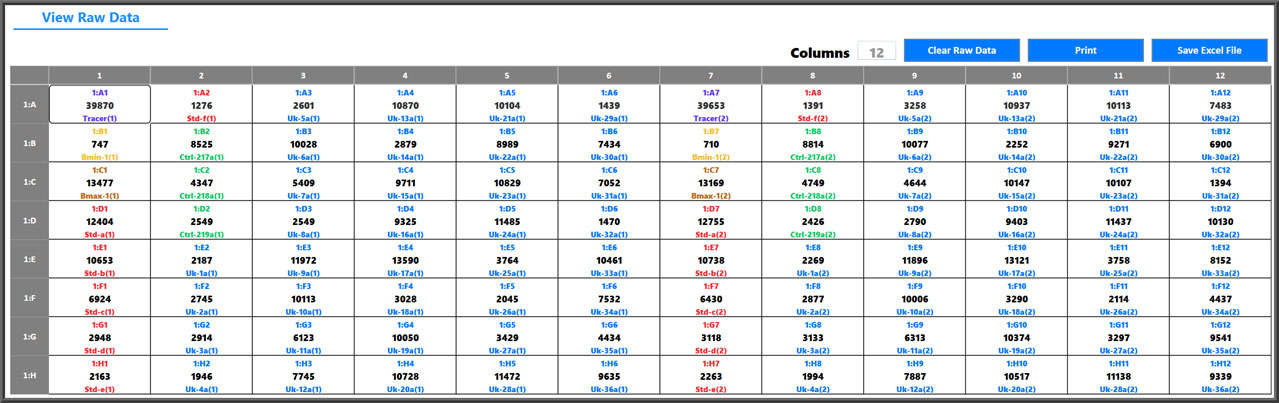
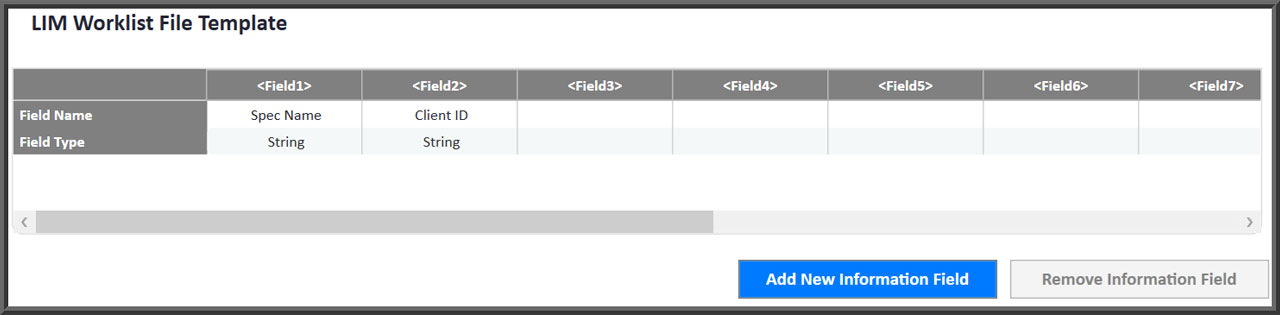
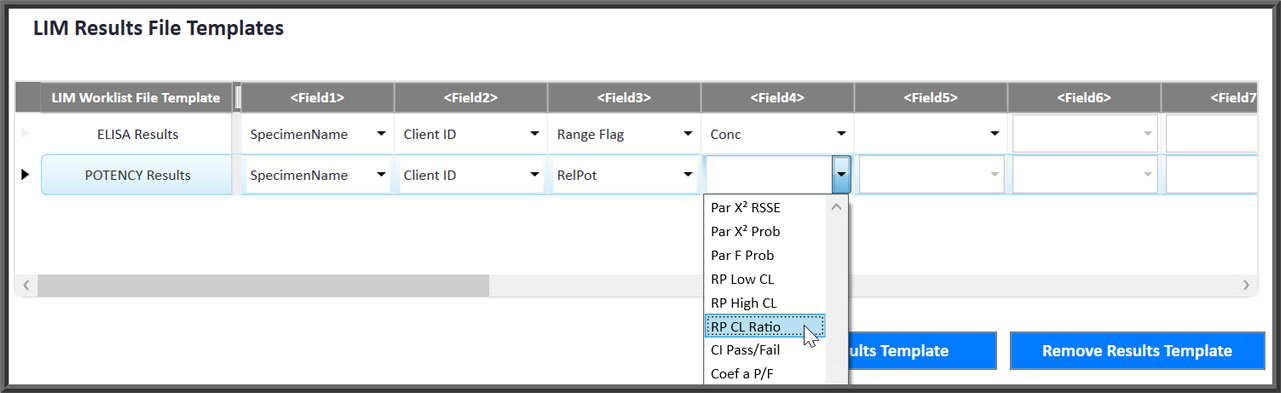
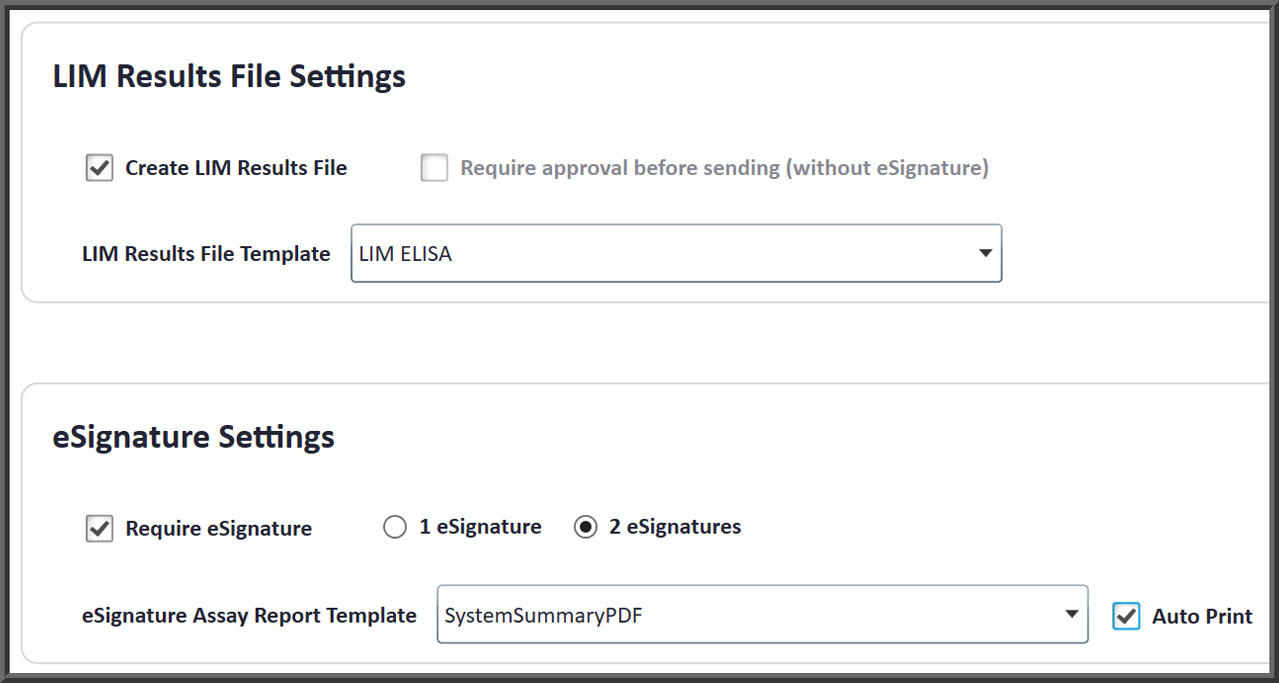
- LIM systems can be interfaced so worklists are downloaded and results are uploaded with project, client and other tracking information easily defined.
- STATLIA MATRIX conforms to GAMP 5 and enables 21 CFR Part 11 regulatory compliance for your GxP bioanalytical laboratory.
- Automated IQ/OQ Validation Package available for complete validation of system and individual detector instruments.
All Your Detectors For All Your Technologies Connected To A Single Advanced System
Easily Interface To Any LIM System
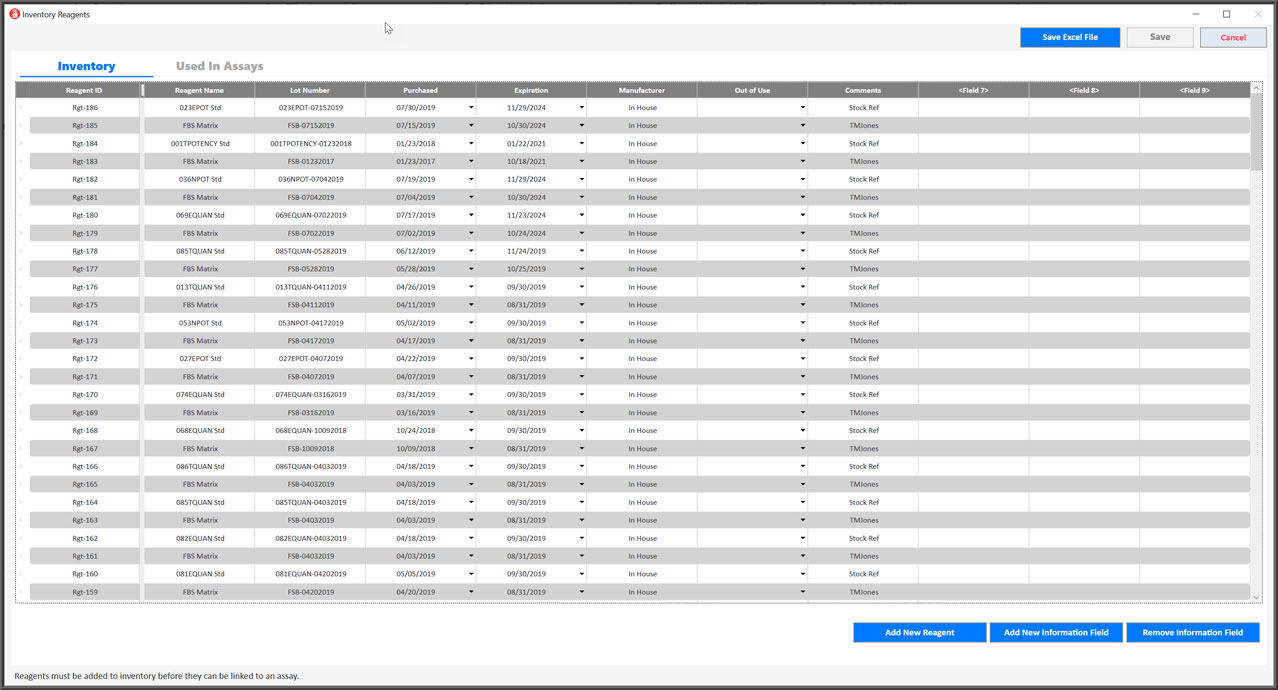
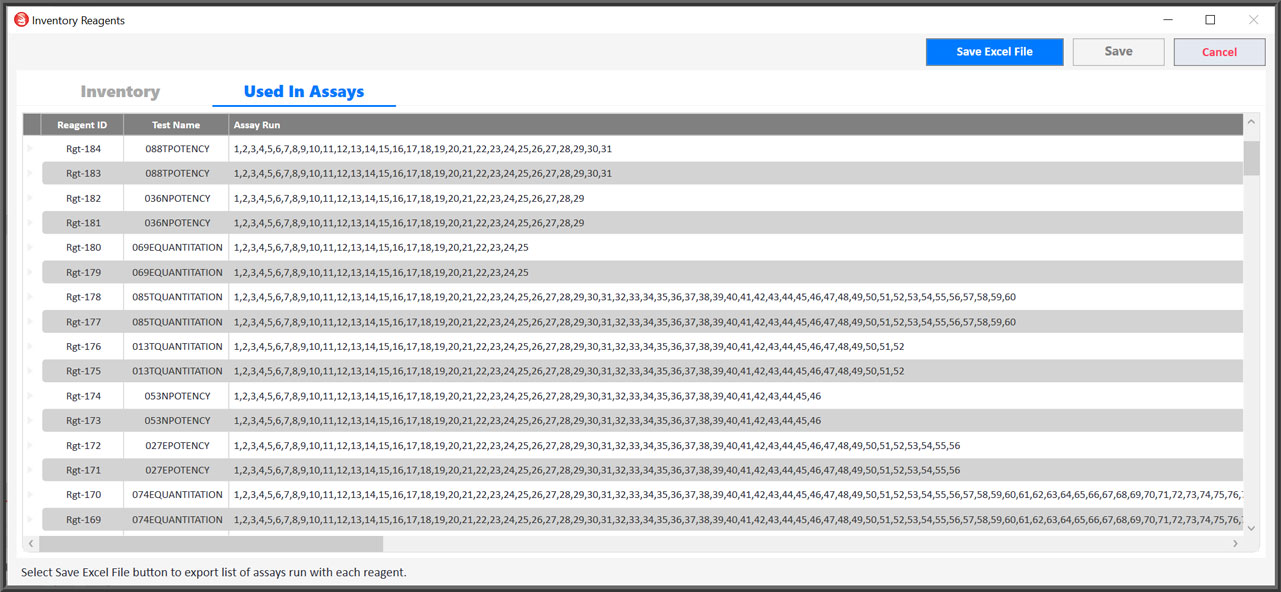
Integrated Control And Reagent Inventory
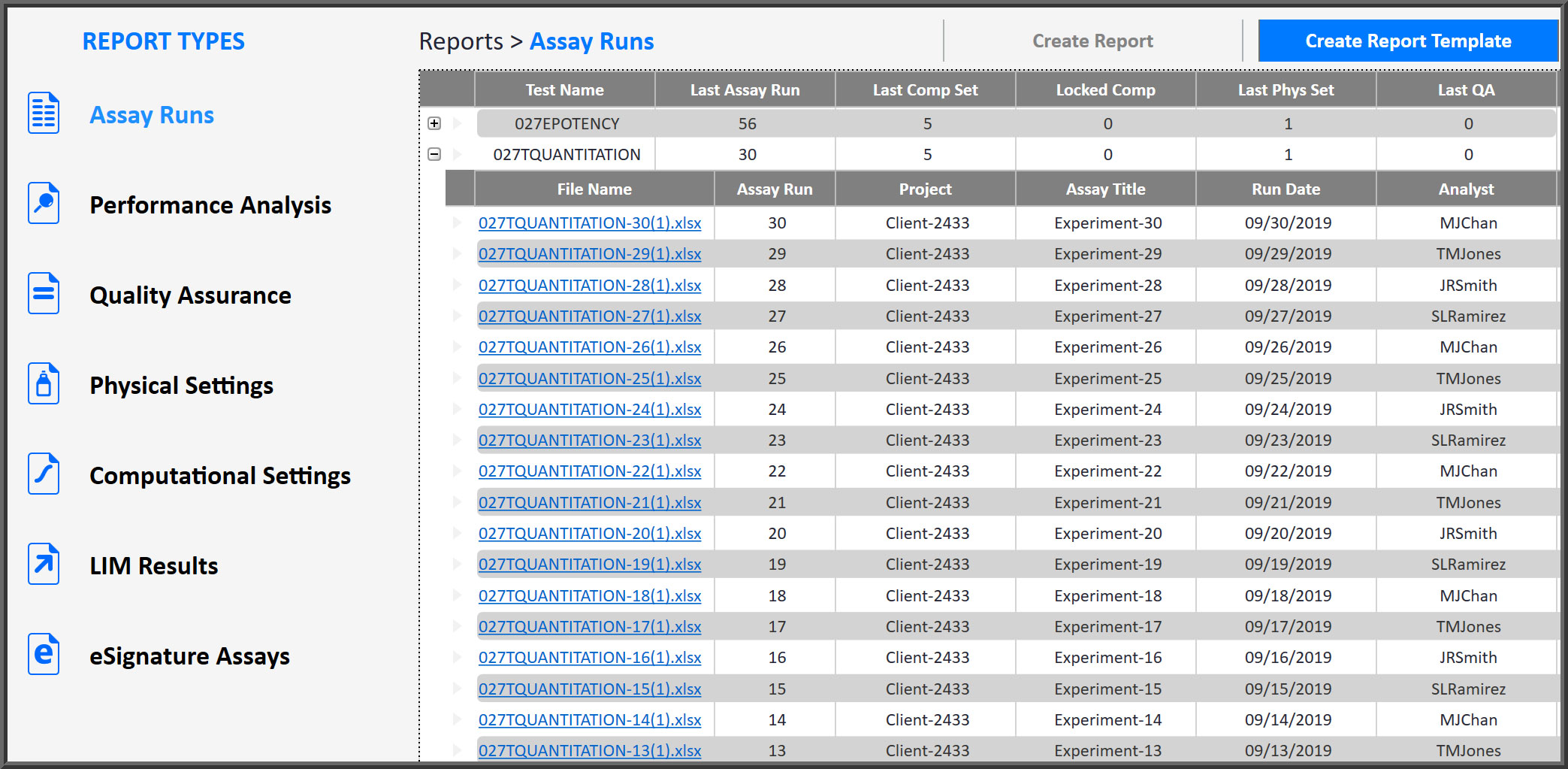
Report Structure Provides Secure, Easy Access
Software Organizes All Reports In One Managed Structure
All reports are secured, sequentially numbered and organized by test for quick, easy access at any time. Reports include: Assay (customizable), Performance Analysis (test behavior), Quality Assurance (test behavior over time), Physical and Computational Settings used for each assay, and LIM Results and eSignature assays waiting for review.