Potency Assays
STATLIA MATRIX performs the three potency methods referenced in the USP 1032, 1033, 1034 and EP 5.3 guidelines.See your data in STATLIA MATRIX.
Relative Potency and Parallelism Testing
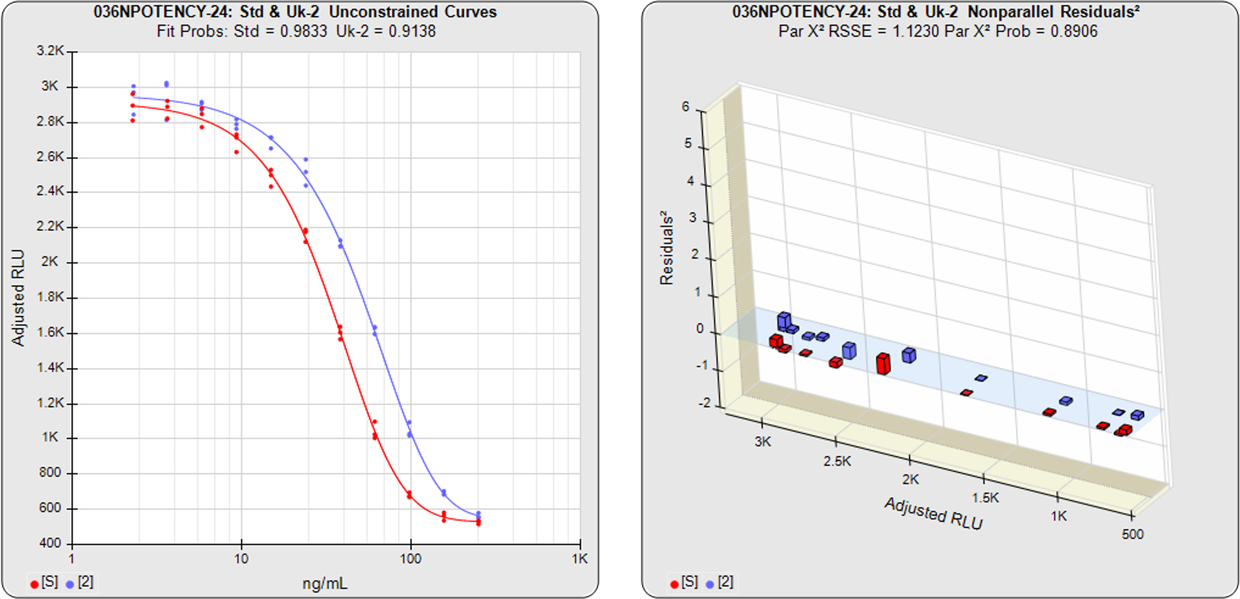
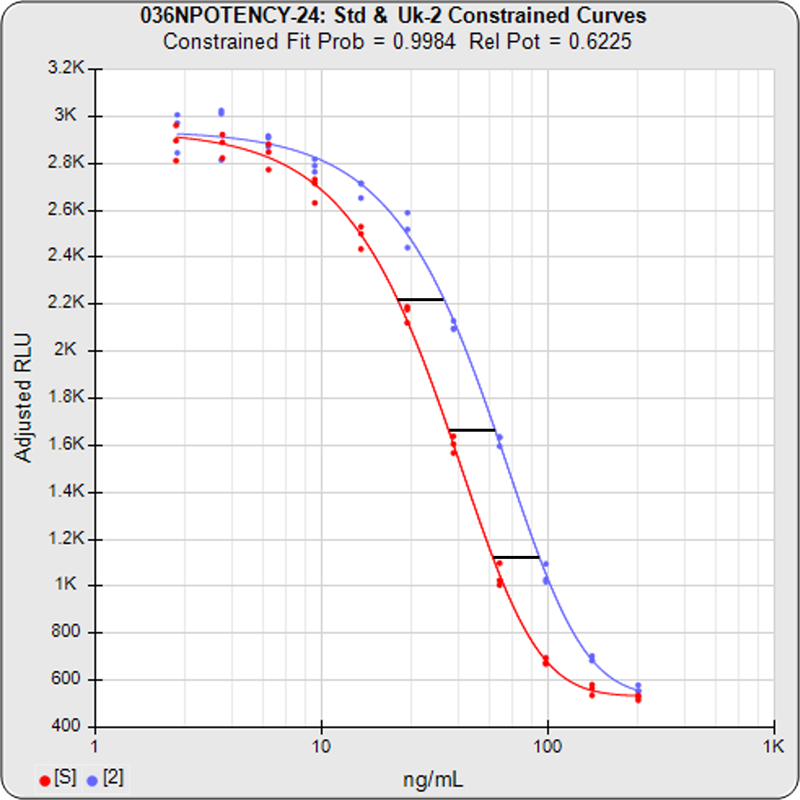 The relative potency of a test sample is the amount of biological activity it produces compared to an equal amount of a reference standard under the same conditions. Relative potency is measured between a series of dilution doses from both materials. The responses from both dilution curves are constrained to fit regression curves having an identical curve shape that provides the best fit for both curves. The distance between the two curves on the log dose axis is the log of the relative potency, and its antilog is the relative potency. This relative potency is equivalent to the ratio of the IC50s between the two curves. Since the two curves are constrained to the same shape, all ICnn ratios will be the same as the IC50 ratio (e.g. black bars between constrained curves in graph on right). Determining the relative potency from unconstrained curves is not accurate since the two curves are never precisely the same shape and ICnn ratios will vary throughout the length of the curves. An estimation of the confidence limits of the relative potency determination between two dilution curves is required to measure its reliability. The log of the upper/lower confidence limits of the relative potency estimate, the RP CL Ratio, provides a stable metric of reliability across a wide range of relative potency values.
The relative potency of a test sample is the amount of biological activity it produces compared to an equal amount of a reference standard under the same conditions. Relative potency is measured between a series of dilution doses from both materials. The responses from both dilution curves are constrained to fit regression curves having an identical curve shape that provides the best fit for both curves. The distance between the two curves on the log dose axis is the log of the relative potency, and its antilog is the relative potency. This relative potency is equivalent to the ratio of the IC50s between the two curves. Since the two curves are constrained to the same shape, all ICnn ratios will be the same as the IC50 ratio (e.g. black bars between constrained curves in graph on right). Determining the relative potency from unconstrained curves is not accurate since the two curves are never precisely the same shape and ICnn ratios will vary throughout the length of the curves. An estimation of the confidence limits of the relative potency determination between two dilution curves is required to measure its reliability. The log of the upper/lower confidence limits of the relative potency estimate, the RP CL Ratio, provides a stable metric of reliability across a wide range of relative potency values.
Testing for parallelism (similarity) between the two dilution curves is a prerequisite for determining the relative potency of two bioactive substances in biological systems. When the two substances are not parallel (not similar), there is no meaningful relative potency between the reference standard and the test sample. Parallelism testing is also used for matrix effects (linearity), cross-reactivity, interfering substances, concentration estimation, and inhibition studies.
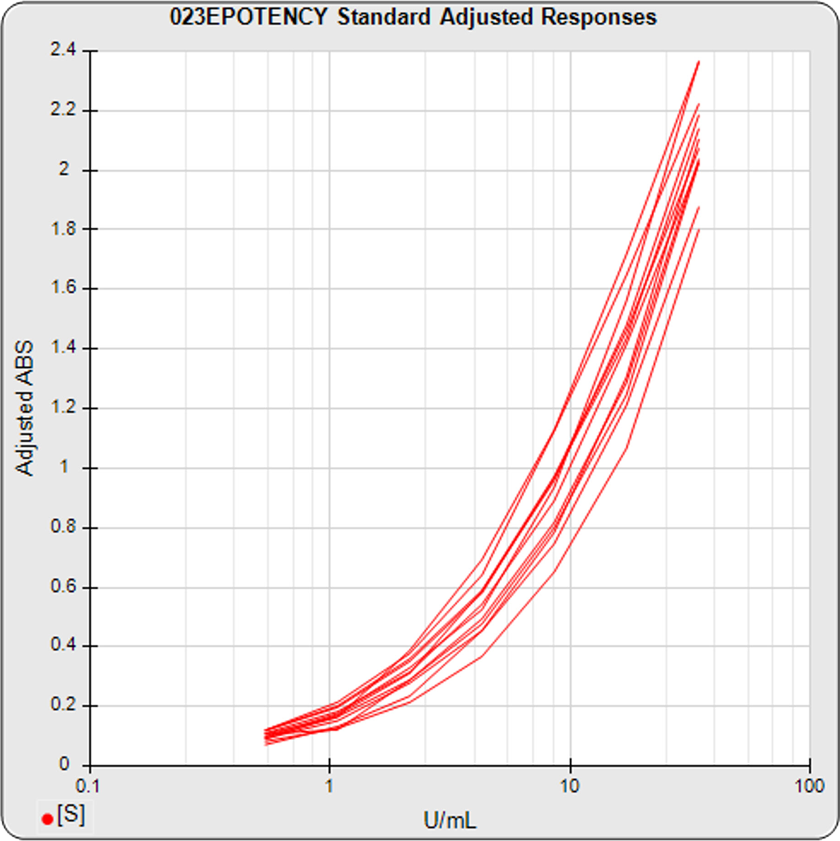
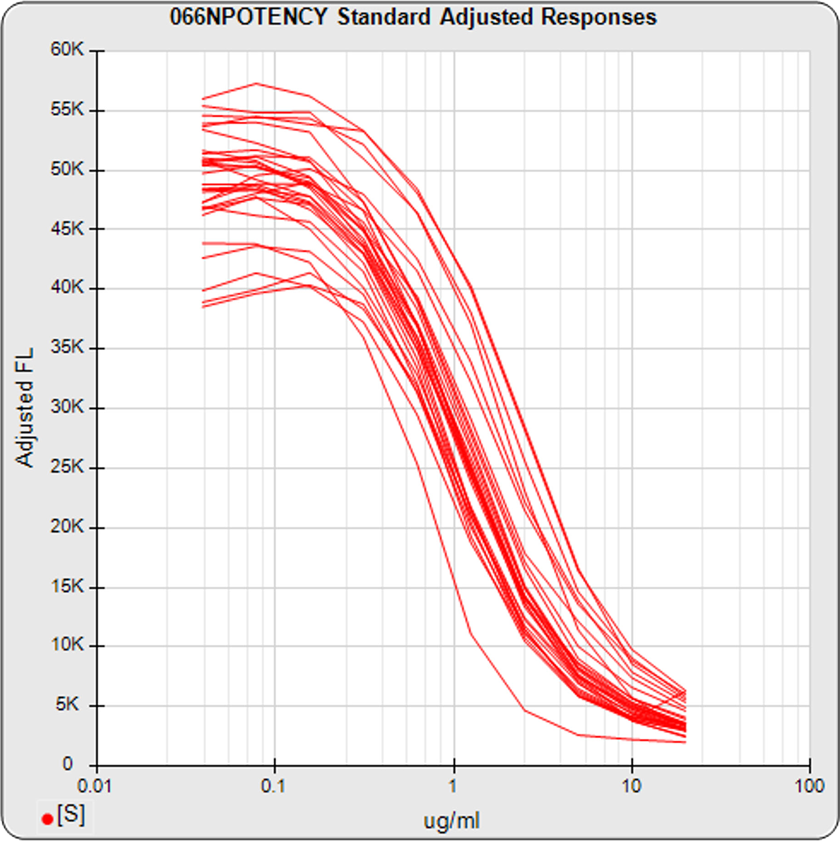
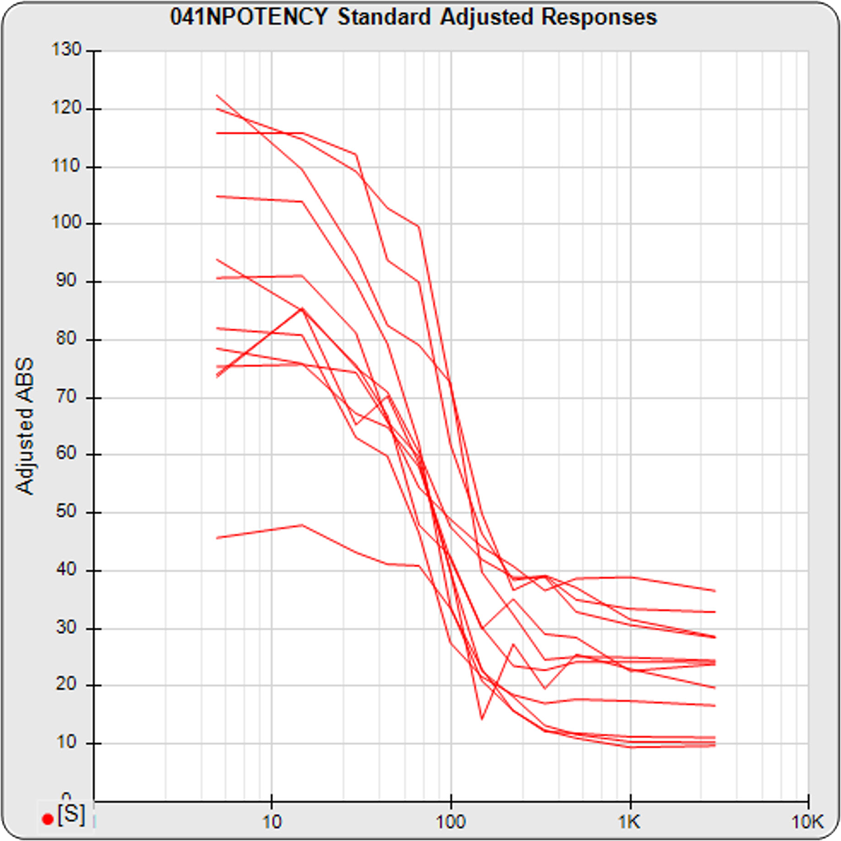
The dilution series can span a full dose response nonlinear curve or a more limited linear set of doses. The advantage of a full dose response curve is that some differences between substances only appear at high or low doses, and relative potency is more accurately determined. But the more limited number of doses needed for a linear comparison is an advantage for animal studies and requires simpler math computations.
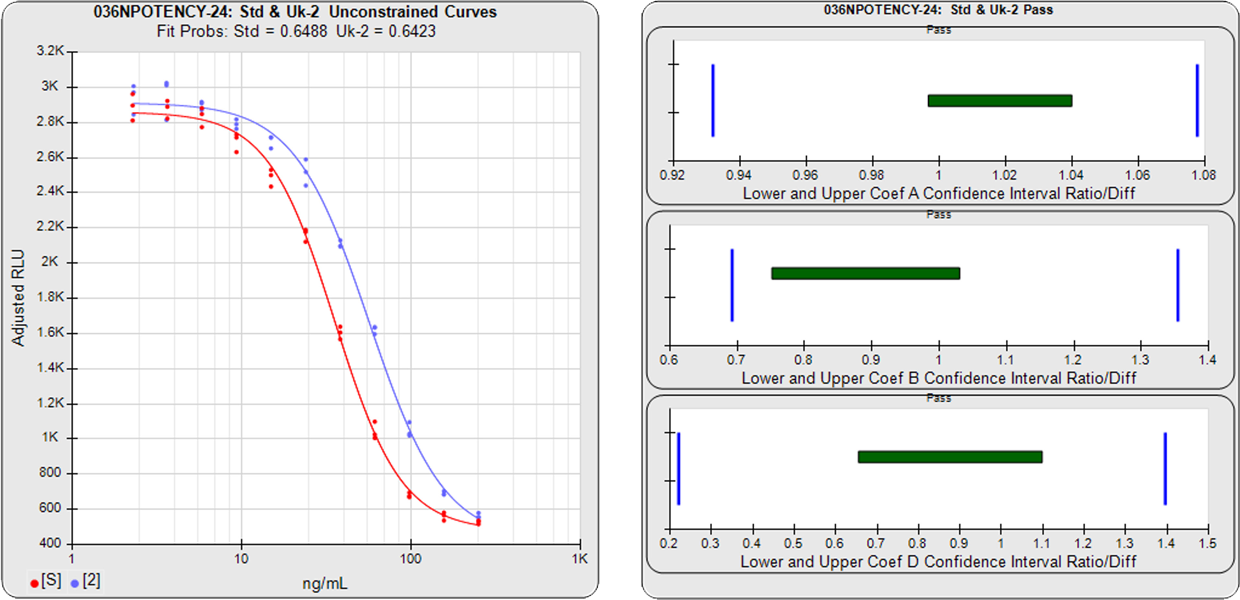
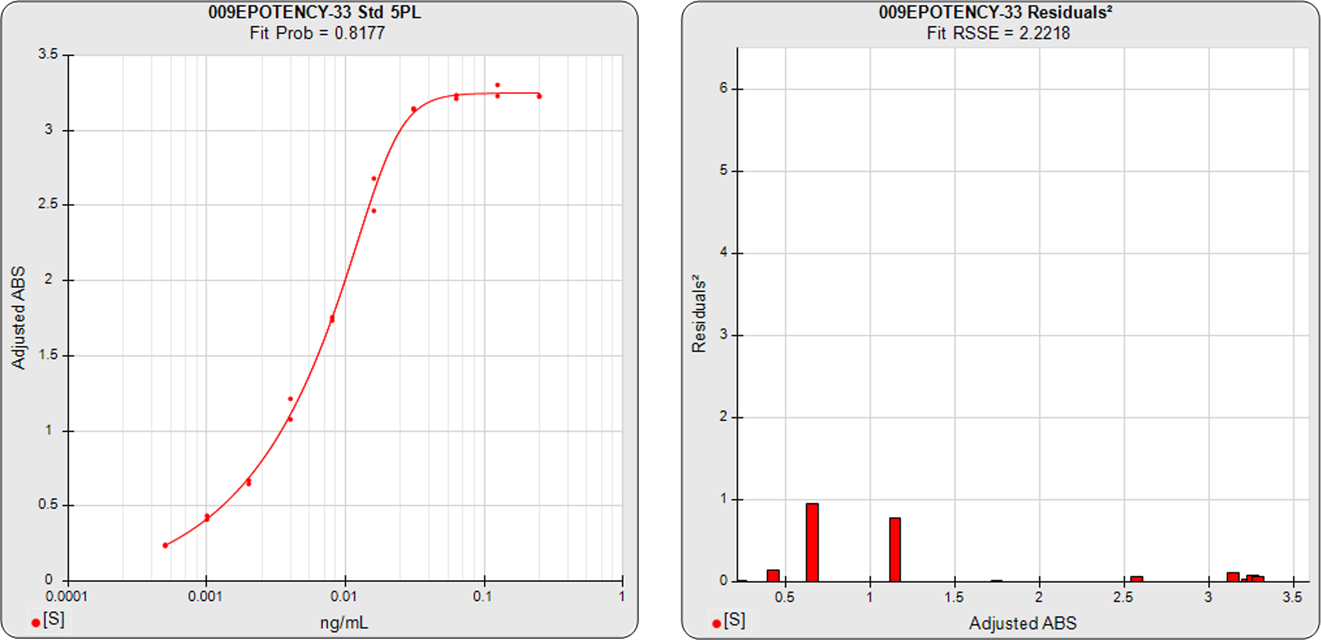
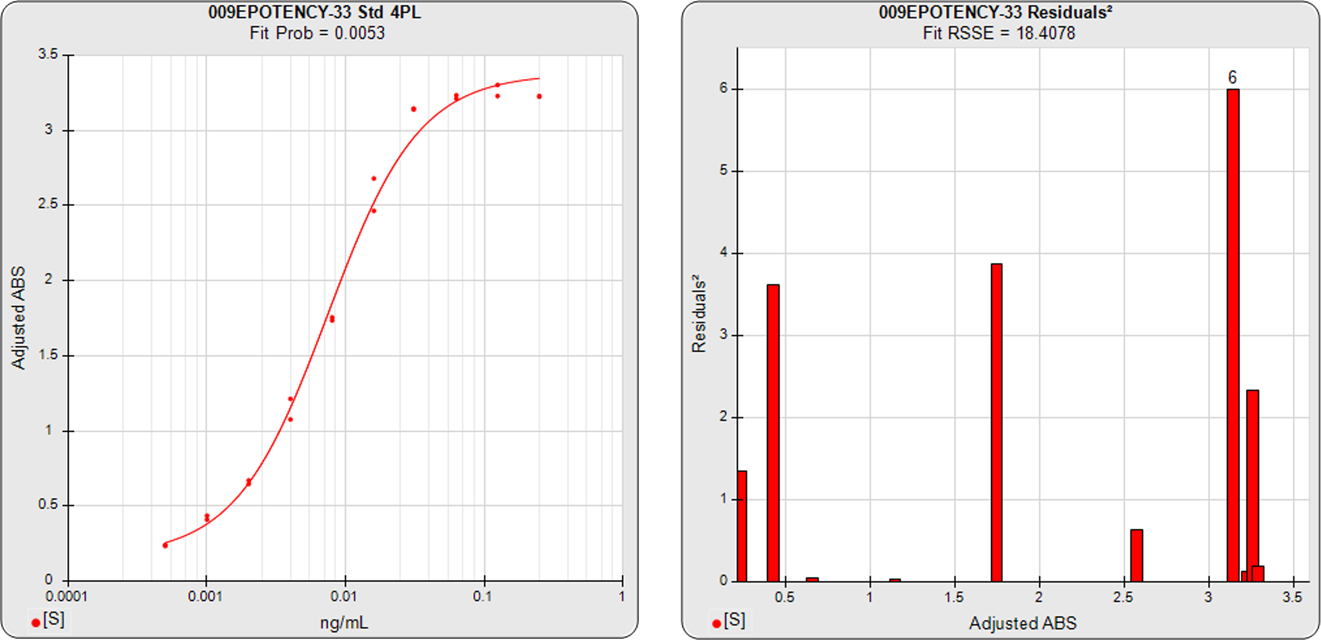
The three main approaches to assessing the parallelism between substances are the Equivalence Method (empirical test), the RSSE (Chi-Square) Method (direct measure of parallelism), and the F Test (hypothesis test). The first method compares the confidence intervals of the regression parameters, and the other two approaches utilize the residual method from regression statistics (also called the extra sum of squares method). All of these methods are cited in the USP 1032, 1033, 1034 and EP 5.3 guidelines and are available in STATLIA MATRIX.
Each Potency Method Uses the Most Accurate Computations In the Industry
Residual Parallelism
Residual Parallelism includes the RSSE(Chi-Square) Method and the F Test and is a measure of the similarity between the weighted residuals2 of the individual dilutions of the two 5PL or 4PL curves or the linear regression of two lines.
With RSSE (Chi-Square) Method, STATLIA MATRIX uses the weighted residuals2 between the observed points and their respective curves to provide an actual measure of the amount of parallelism between the two curves or lines to determine parallelism.
Confidence Interval Parallelism
Confidence Interval Parallelism (also called the Equivalence Method), is a measure of the similarity between the asymptotic end points plus the slope of the inflection point of the two curves. For linear regression, the similarity of the slopes is measured.
STATLIA MATRIX determines the confidence interval limits of each curve or line’s coefficients and then compares them to determine parallelism.
Even Your Most Difficult Potency Assays Are Easily Handled in STATLIA MATRIX
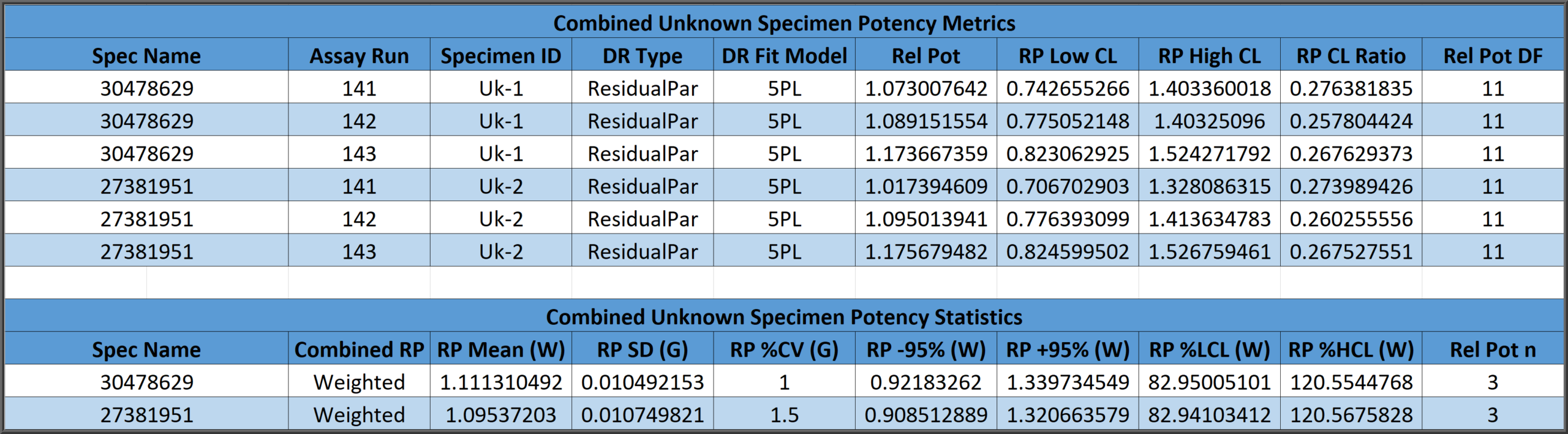
Test Sample Relative Potencies from Multiple Assays Combined
All relative potencies of a test sample determined in previous assays are combined automatically from the database in assay reports. The three methods cited in USP 1034 guidelines for combining multiple relative potencies from independent determinations establish a reportable potency and 95% confidence intervals. These three methods determine a simple arithmetic mean and confidence limits (A), a geometric mean and confidence limits from the logarithms of the relative potencies (G), or a weighted mean computed from the logarithms weighted by high and low confidence limits of each relative potency determination distributed as a homogeneous or heterogenous population (W). Failed test sample results can be suppressed.
Control dilution curves can also be assayed. Control relative potencies are compared to reference statistics from your pooled assays or tolerance ranges set by your laboratory for Pass/Fail determinations.
Your Previously Run Assays Show If Your Potency Test is Stable and Reliable
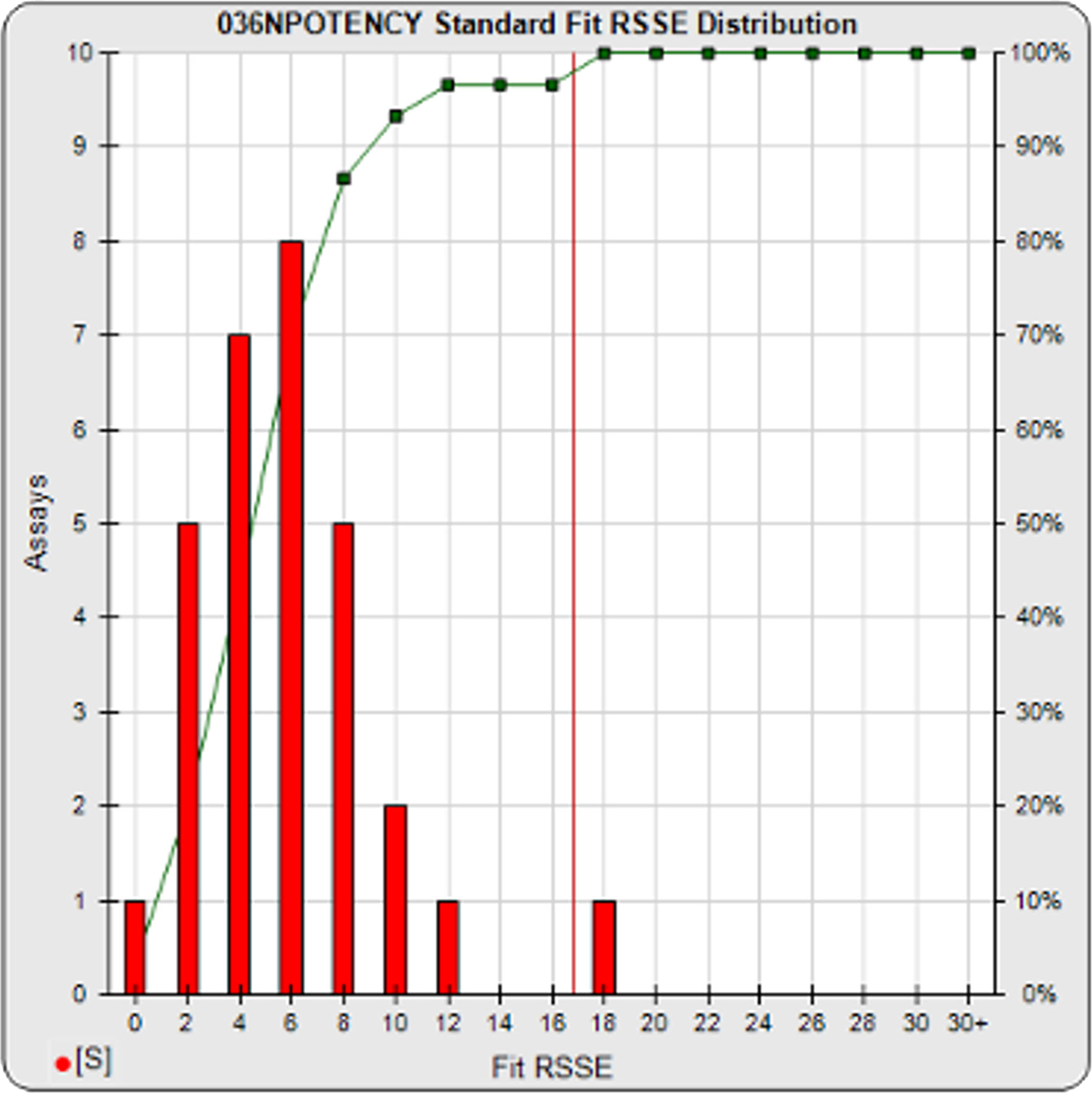
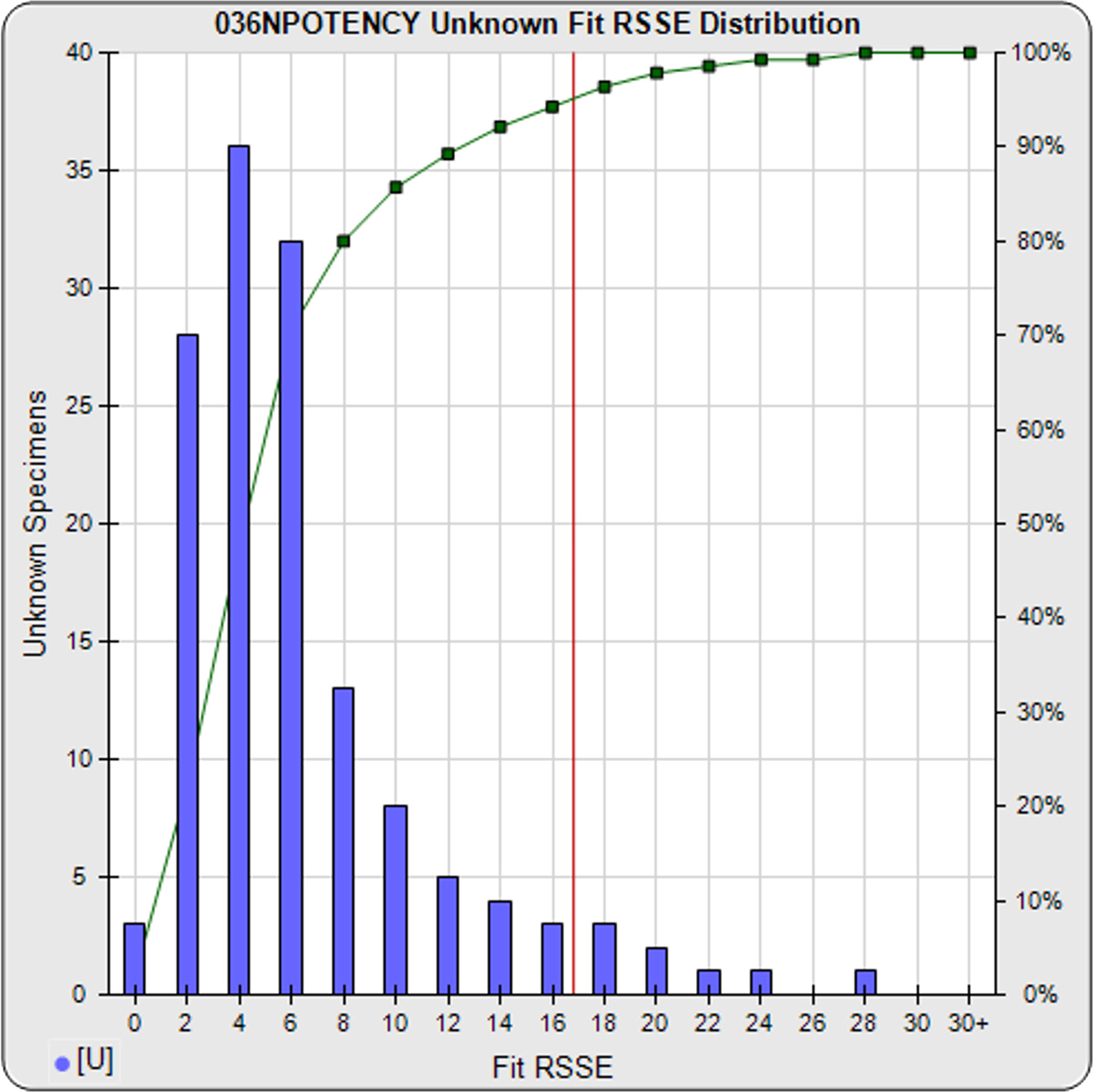
Use STATLIA MATRIX graphs and metrics of the data from a pool of your previously run assays to determine the stability and reliability of your potency test. The standard and unknown Fit RSSE distribution’s show the suitability of the test for reliable potency determinations. The red line shows the acceptance thresholds, either computed automatically by the software or set by the laboratory. The cumulative percentage of curves at each level of Fit RSSE is plotted on the right axis in green.
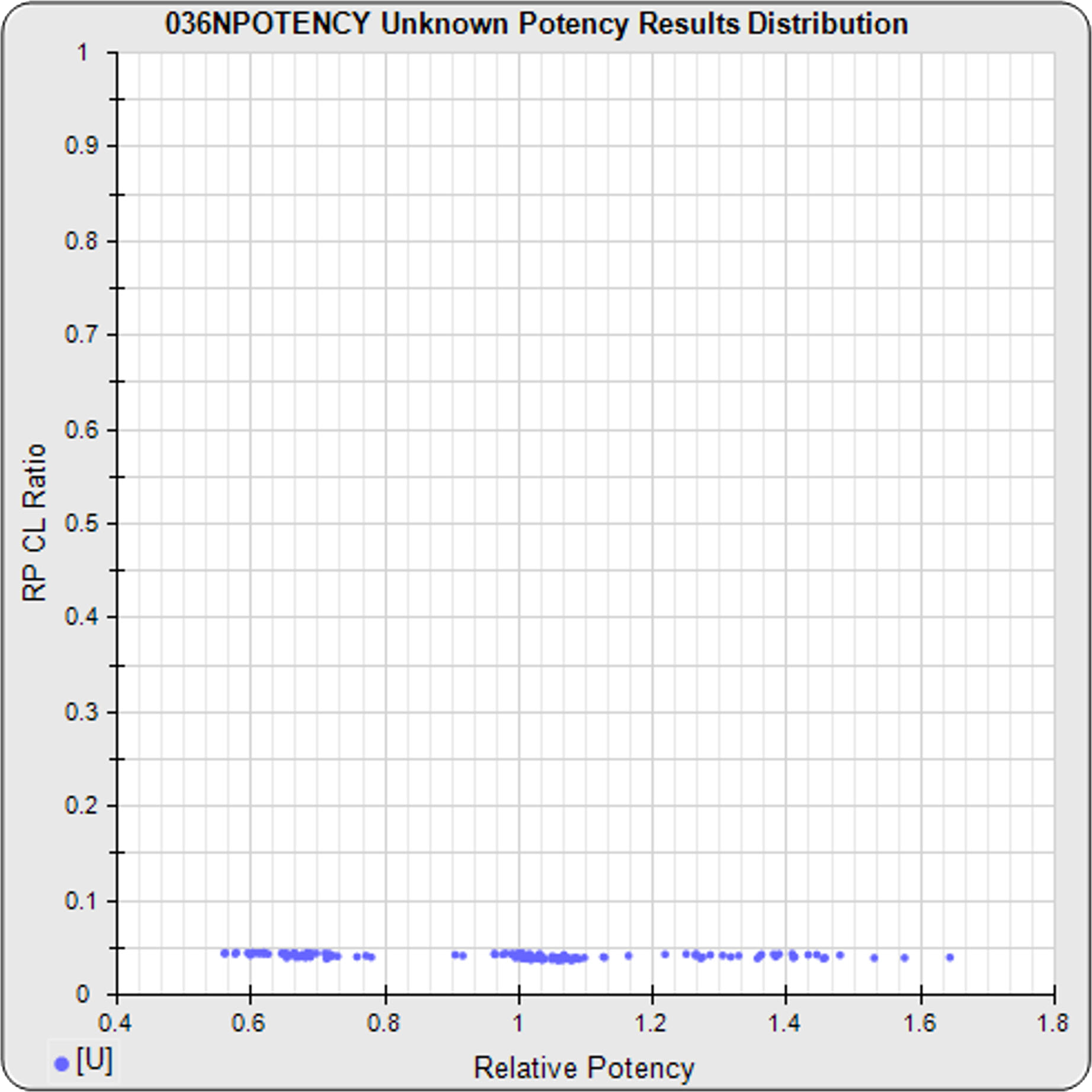
The relative potencies (X-axis) versus their confidence limit ratios (Y-axis) are plotted for all unknowns (blue dots) in the pooled previously run assays. The log of the upper/lower confidence limits of the relative potency estimate (RP CL Ratio) show the reliability and stability of the relative potency results across the range of values tested. A rise in the RP CL Ratio at either end shows the limits of reliability for relative potency determinations.